What is ozone
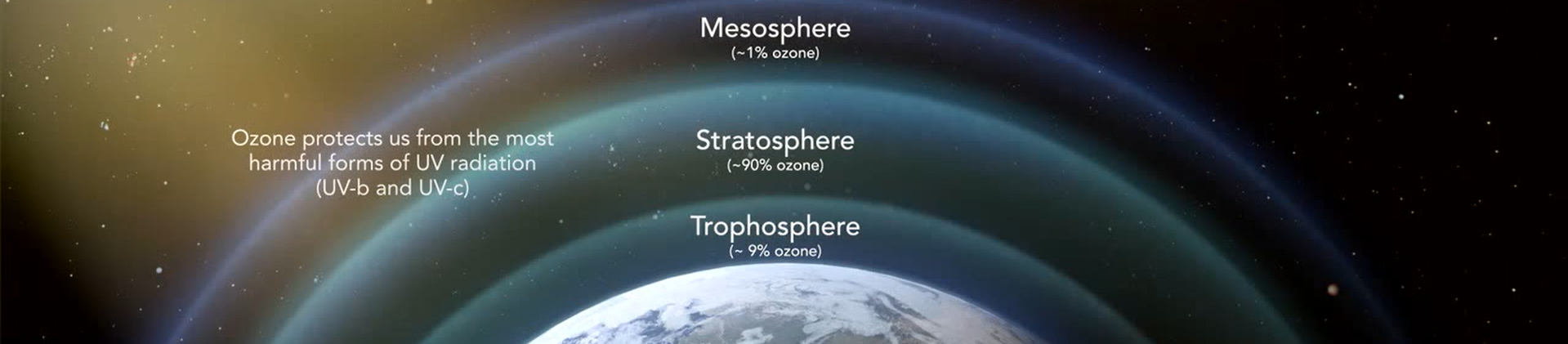
Ozone is a gas composed of three atoms of oxygen (O3). Ozone is the Earth’s Natural Sunscreen and occurs both in the Earth’s upper atmosphere and at ground level.
Ozone is naturally occurring and is created when ultraviolet light from the sun hits an O2 molecule producing monatomic O. The monatomic oxygen temporarily bonds with an O2 molecule to produce O3. In nature the air we breathe contains 10 to 100 parts per billion ozone and this is enough to naturally discourage the growth of many micro-organisms. Overall the atmosphere contains about 600 ppb ozone with concentrations higher in the upper atmosphere where UV light is much more abundant.
Ozone is a form of oxygen. As a strong oxidant, ozone is a type of natural sterilizer, which could effectively oxidize (decompose) organic matter. Unlike the usual chemical disinfectant, it neither causes any negative side effects, nor corrosive. In fact, ozone is an environmental friendly sterilizer that reforms into oxygen molecules after the sterilization process. Ozone in the atmosphere is usually produced via ultraviolet ray released by the Sun and through lightning strikes. This explains why we could always feel like there’s fresh air after thunderstorms, as ozone has been produced during the storm and has cleansed the air in the atmosphere.
Ozone is very useful for industries which requires high level of hygienic and sterilizing standards, such as food safety, commercial laundry, medical, water treatment industries and etc. Ozone is also listed as the approved sterilizer by the US Environmental Protection Agency (EPA), and has been utilized across the globe for more than a century. Many running water treatment in developed countries and most packaged drinking water utilize ozone to sterilize.
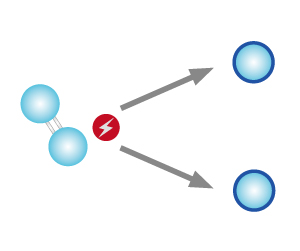
How is Ozone Formed
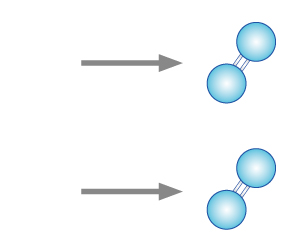
| STEP 1 High energy (from ultraviolet ray or lightning) splits oxygen molecules into oxygen atoms. |
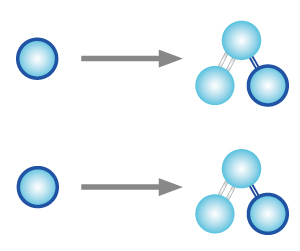
STEP 2 The free oxygen atom react and form weak bond with another oxygen molecule to create an ozone molecule. |
 |
 |
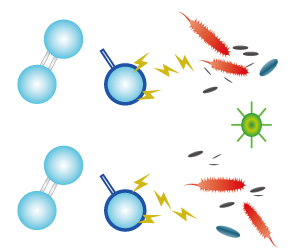
| STEP 1 As ozone molecules react with organic molecules, the oxygen atom with the weak bond will be released, thus oxidising the organic matter. |
STEP 2 Ozone molecule will reform into oxygen molecule (oxygen) after oxidising the organic matter. |
 |
 |
How long can ozone last?
Ozone can have a half life as low as 15 minutes in 25 Temp (°C). Let’s say that we have treated a greenhouse for 24 hours and the concentration has built up to 160 ppm. After we turn off the O3 system, in 15 minutes the concentration of O3 will go down to 80 ppm. After half hour, its down to 40 ppm, 45 minutes we’re down to 20 ppm. If we turn on a fan and open the windows, the concentration goes down much more rapidly. With ventilation and natural decay, half life may short as few min in natural environment.
Regulatory approval
Regulatory agencies Food and Drug Administration (US-FDA), United States Department of Agriculture (USDA), Food Safety Inspection Service (FSIS), Federal Food, Drug, and Cosmetic Act (FFDCA), The Antimicrobial Regulation Technical Corrections Act (ARTCA) have approved ozone in the treatment, storage and processing of foods.
What things do ozone eliminate or reduce?
Pesticides, bacteria, virus, mold, fungus, many chemicals and organic smells.
Landmarks in the approval of ozone
In 1982 the FDA declared ozone a GRAS treatment for bottled water.
In 1997 the FDA was presented with a study that affirms the history and safety of ozone use with foods.
Is ozone harmful – are the long term effects?
There are a few websites out there that say that Ozone is dangerous and should not be used. These naysayers aren’t professional and are not aware of the US-EPA stance on Ozone, its properties and applications. With that said, Ozone should be treated with respect and should not be over inhaled.
“March 12, 1975 – FDA recognized ozone treatment to be a Good Manufacturing Practice (GMP) for the bottled water industry. The minimum ozone treatment for GMP is “0.1 part per million (0.1 mg/l) of ozone in water solution in an enclosed systems for at least 5 minutes.” Code 21 of Federal Regulations, Section 129.80 d.4 Federal Register 11566, 12 March 1975
What are the applicable regulations for ozone offgas
OSHA regulates two levels for ozone off gas exposure. They are 0.1 ppm time weighted average (TWA) for an 8-hour day, and 0.3 ppm for 15 minutes.
The way to generate ozone
Ultraviolet Light (UV Ozone Generator)
Raw Material: Air Filter; Consumable: UV Lamp
Using ultraviolet ray with certain wavelength (185mm) to radiate through the oxygen molecule, oxygen molecules will be broken and produce ozone molecules, a portion of the photons (UV light) hit and excite the O2 molecule to the point where the bond breaks and monatomic oxygen is produced. This type of ozone production are commonly used in cupboard disinfection process, and the ozone product by the UV method has not more than 0.3% purity.
Corona Discharge Method
Raw Material: Atmosphere Air or Pure Oxygen; Consumable: Air Desiccant, Air Filter, Oxygen Production Machine.
The principle of corona discharge method for producing ozone is to place a dielectric body parallel between two parallel high-voltage electrodes and maintain a certain discharge gap. When high-voltage alternating current is applied between the two poles, a uniform blue-violet is formed in the discharge gap. Corona discharge, when air passes through the discharge gap, oxygen molecules are excited by electrons to obtain energy, collide with each other, and polymerize into ozone.
The corona discharge method is currently the most common form of ozone generation, oxygen is pushed through high voltage plates and is “electrified” where O2 molecules are pulled apart, and later recombine to produce O3. and the purity of ozone generated by the corona discharge method is up to 6%. The corona method produces ozone in the corona air (20%) while coronarating the nitrogen in the air (78%) and producing by-product nitrogen oxides (NOx), while corona discharge using pure oxygen. The law does not produce nitrogen oxides.

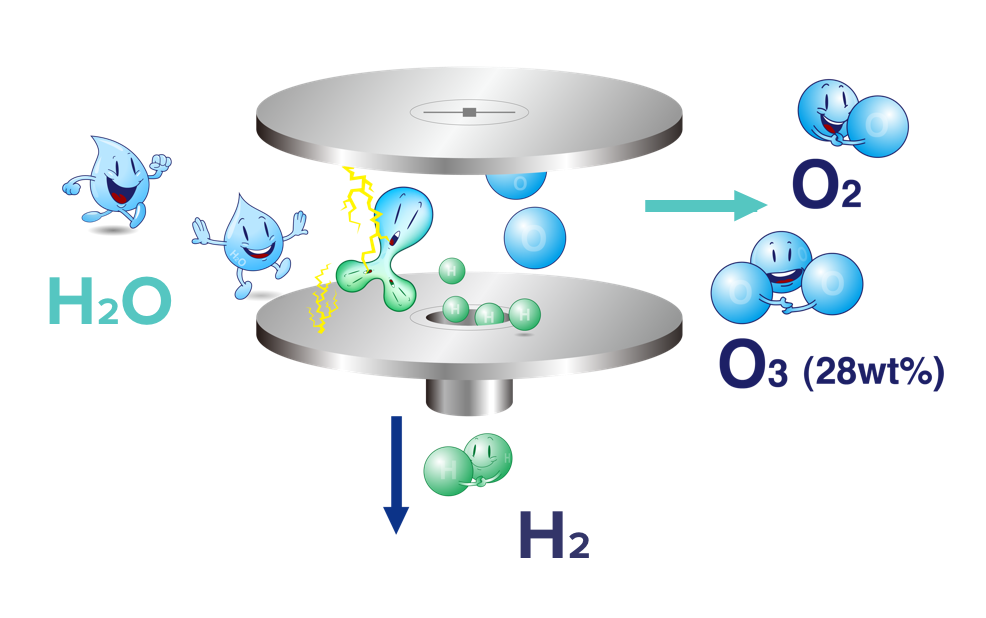
Electrolytic Ozone Generation (EOG)
Raw material: Water;Consumable: Pure water
The principle of membrane electrode electrolysis to produce ozone is to use low-voltage direct current to conduct positive and negative two-pole pure water of solid-state membrane electrode. Water is separated into hydrogen and oxygen molecules in the form of proton exchange at a special anode interface, and hydrogen is directly from the cathode. Emission, oxygen molecules are excited by electrons generated by high-density currents at the anode, and are polymerized into ozone.
The purity of ozone produced by electrolysis is about 20% or more. The by-products of the production of ozone by electrolysis are oxygen and hydrogen, and no nitrogen oxides (NOx), and air humidity is not considered.
iEOG Electrolytic Ozone Generator Principle
Difference Between Ozone Gas & Ozonated water
Ozone gas is a natural oxidant (bactericide), but the gas has no directionality and cannot sterilize the surface of the object. If water is used as a medium and the ozone gas is efficiently dissolved in water, the directionality of the ozone gas can be achieved. Instead of many chemicals, the surface of the object is disinfected.
According to the OSHA (Occupational Safety and Health Association) regulations in the United States: the average ozone concentration exposed for 8 consecutive hours in the working environment should not be higher than 0.1 ppm to prevent respiratory tract sensitivity and cough, so it is necessary to control the air in the application of ozone gas. Concentration and ventilation to maintain the environment, however, ozone gas has been used so far, there has never been a case of death due to ozone.
Importance of Ozone Gas Purity
The amount of gas that can be dissolved per unit of water molecules is limited. Therefore, the purer and less impurity source of ozone gas will determine the concentration of ozone water after dissolution. If the source of ozone gas is not pure, other gases will also be Dissolved in water. The purity of ozone gas generated by Biotek iEOG is high, and ozone water does not contain other substances except ozone gas and oxygen.
The Benefits of High Purity Ozone
For example, in the carbonated beverage industry, since carbon dioxide needs to be driven into water by pressure, when it is necessary to dissolve more carbon dioxide, the purity of the gas in the water source is relatively important, and the more impurities, the relative influence of the efficiency of carbon dioxide dissolved into the carbonated beverage.
iEOG can produce high-purity ozone gas. Due to the limited gas per unit area of water, the use of high-purity ozone gas (no nitrogen, less oxygen) can greatly increase the efficiency after mixing, because more effective ozone can be dissolved. In the water, in addition to the high concentration, the gas aeration is also small, achieving the advantages of safety and low post-treatment cost.
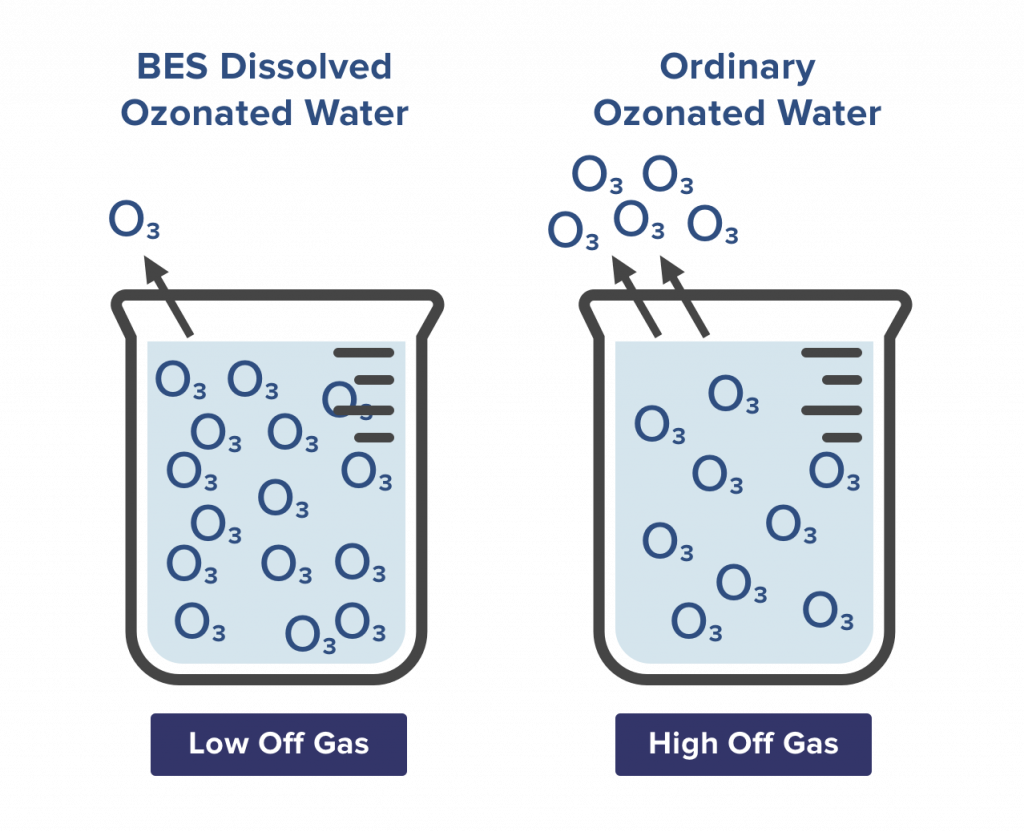
BES High Concentration, Low Aeration Ozone Water
BES has a number of patented dissolving technologies to dissolve ozone gas in water stably. The aeration amount meets safety regulations and generates high concentration of dissolved ozone water. The dissolved high concentration ozone water can effectively replace chemical disinfectant for human body and environment.
More friendly.
BES Technology & Products – Safe Aeration that Exceeds International Standards
Continuous use in 15 minutes, the average concentration in the air is less than 0.3 ppm.
Intermittent use within 8 hours, the average concentration in the air is less than 0.1 ppm.